A dynamic and adaptable approach
In the ever-evolving landscape of clinical trials, the concept of hybrid sourcing is taking center stage, offering a dynamic and adaptable approach to sourcing essential supplies. At its core, hybrid sourcing combines two distinct strategies: direct sourcing from manufacturers and sourcing from the open market. This innovative approach is gaining traction for its ability to optimize efficiency and flexibility in the clinical trial supply chain.
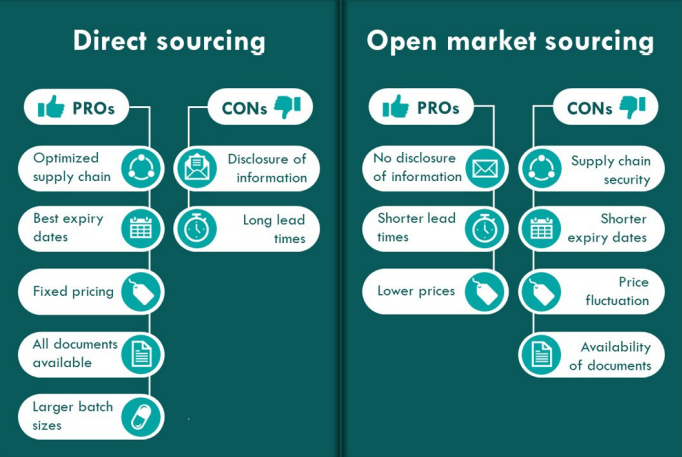
Direct sourcing and open market sourcing
Direct sourcing from manufacturers ensures a streamlined and controlled supply process. It provides pharmaceutical companies with a direct line to the source, enabling them to negotiate favorable terms, maintain stringent quality control, and secure a steady supply of critical materials. The pros of this sourcing method are; the best expiry dates, all documents available, and larger batch sizes.
On the flip side, sourcing from the open market introduces an element of flexibility. It allows companies to tap into a broader marketplace, potentially finding cost-effective alternatives and mitigating supply chain disruptions. This strategy is especially beneficial when rapid procurement is essential (shorter lead times).
Strategically blending
The synergy between these two approaches empowers clinical trial companies with the agility to adapt to ever-changing circumstances. By strategically blending direct sourcing and open market procurement, companies can optimize costs, ensure supply chain resilience, and ultimately enhance the efficiency and success of their clinical trials. Hybrid sourcing is not just a buzzword; it’s a strategic imperative for modern clinical trial management.
BMclinical excels in delivering hybrid sourcing solutions for clinical trials, leveraging both direct manufacturer sourcing and open market procurement. This strategic approach ensures cost-effectiveness, quality control, and supply chain resilience, making BMclinical a leader in optimizing clinical trial supply chains.

